Abstract
All transcriptionally active and rapidly dividing cells require high amounts of pyrimidines for DNA replication and repair and RNA synthesis. The only two known mechanisms by which cells can acquire pyrimidines are the de novo pyrimidine biosynthesis pathway and the pyrimidine salvage pathway. The rate-limiting enzyme in de novo synthesis is dihydroorotate dehydrogenase (DHODH), a critical metabolic enzyme that converts dihydroorotate (DHO) to orotate, which is then converted to the pyrimidine precursor uridine monophosphate (UMP). The rate-limiting enzyme in the salvage pathway is uridine-cytidine kinase 2 (UCK2), a ribonucleoside kinase that converts uridine to UMP and cytidine to cytidine monophosphate (CMP). Under physiologic conditions, salvage pathway substrates are limiting, and many cancer types, most notably acute myeloid leukemia (AML), are highly dependent on DHODH activity for proliferation and survival. Based on these observations, several DHODH inhibitors have been developed as anti-cancer therapeutics and have been tested clinically. Unfortunately, to date, these drugs have failed to show significant anti-cancer activity in early-phase clinical trials.
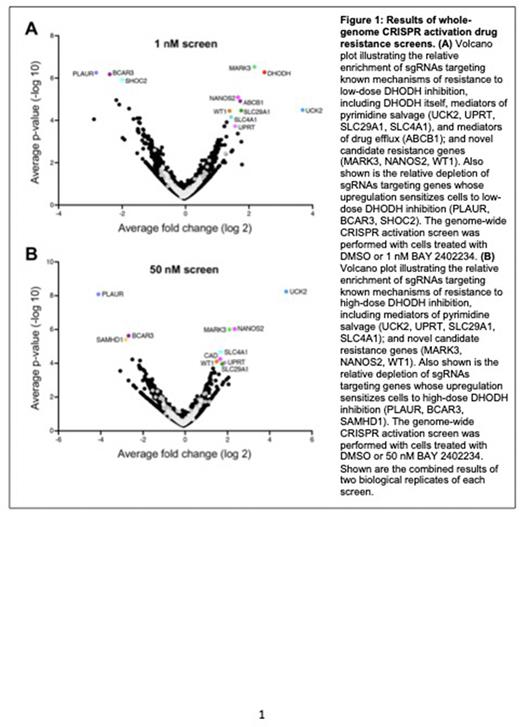
In order to understand how leukemia cells that are highly sensitive to DHODH inhibitors can bypass their dependence on DHODH activity, we conducted positive-selection whole-genome CRISPR-activation drug resistance screens in TF-1 human AML cells using BAY 2402234, a highly potent and highly specific DHODH inhibitor. In preliminary studies, we found that 1 nM BAY 2402234 fully inhibits TF-1 cells whereas TF-1 cells that overexpress high levels of DHODH require 50 nM BAY 2402234 to achieve the same effect. Importantly, the effects of 1 nM and 50 nM BAY 2402234 are fully rescued by addition of uridine to the culture media, demonstrating that the anti-leukemic activity of BAY 2402234 is on-target at both drug doses.
To perform the drug resistance screens, we stably expressed nuclease-deactivated Cas9 fused to a transcriptional activator (dCas9-VP64) in TF-1 cells and then infected the cells with the human whole-genome Calabrese sgRNA lentiviral library. We then passaged the cells in media containing vehicle control (DMSO), 1 nM or 50 nM BAY 2402234 for 18 days, collecting cells on day 0 and day 18 of drug treatment. Genomic DNA was then extracted and was subjected to next generation sequencing to assess the relative abundance of each sgRNA in each sample. We reasoned that activation of genes that enhance de novo pathway activity at the level of, or downstream of, DHODH would promote resistance to 1 nM BAY 2402234 but not 50 nM BAY 2402234, whereas activation of genes that act through de novo pathway-independent mechanisms would promote resistance to both 1 nM and 50 nM BAY 2402234.
As predicted, sgRNAs targeting DHODH conferred resistance to 1 nM but not 50 nM BAY 2402234 whereas sgRNAs targeting UCK2 conferred resistance to both 1 and 50 nM BAY 2402234 (Figure 1). A number of other known salvage pathway genes scored in both screens, including uracil phosphoribosyltransferase (UPRT) and the nucleoside transporters SLC4A1 and SLC29A1. Unexpectedly, the screens also identified carbamoyl-phosphate synthetase 2/aspartate transcarbamoylase/dihydroorotase (CAD) as a potential mechanism of resistance to DHODH inhibition. CAD is a trifunctional multi-domain enzyme that acts upstream of DHODH, catalyzing the first three steps in de novopyrimidine biosynthesis. How upregulation of CAD activity can bypass the requirement for DHODH activity is not clear, although CAD is a very large enzyme complex about which very little is known besides its de novo pyrimidine synthesis activity. The screens also identified several genes that are not known to play a role in pyrimidine metabolism as potential resistance mechanisms. These include the serine-threonine kinase MARK3, the serine-threonine phosphatase CTDSPL2, and the RNA-binding protein NANOS2. Finally, the screens identified several genes whose upregulation sensitized cells to BAY 2402234, including the urokinase receptor PLAUR, the adaptor protein BCAR3, the scaffold protein SHOC2, and the deoxynucleoside phosphohydrolase SAMHD1.
Studies are currently underway to validate the results of the CRISPRa screens and elucidate the mechanisms by which the candidate resistance genes help cells to bypass their requirement for DHODH activity.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal